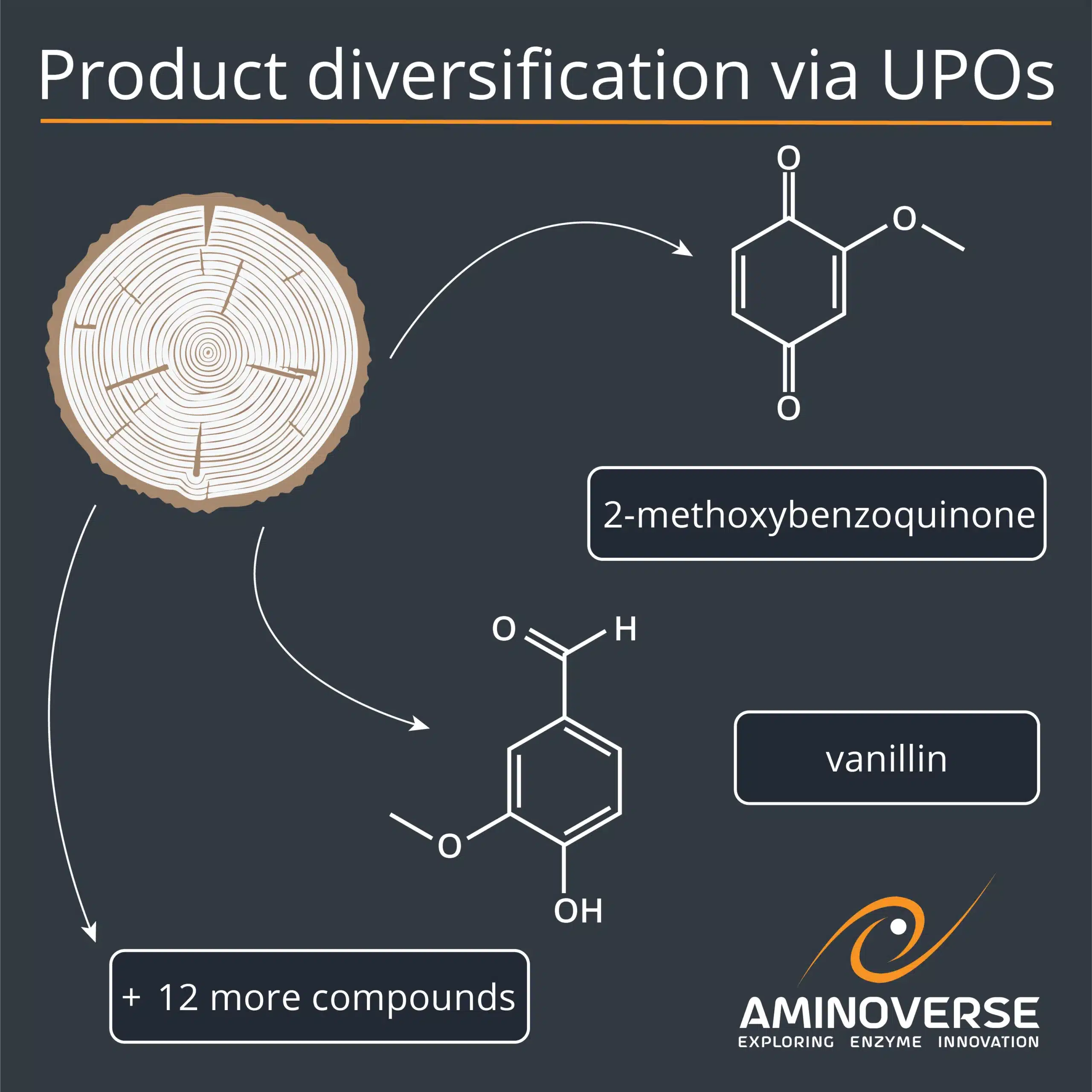
UPO promiscuity unlocks diverse compounds
The recent work of Essi Rytkönen, Juha Rouvinen and Janne Jänis illustrates the catalytic breadth of unspecific peroxygenases (UPOs). Using two lignin model compounds, the team applied Aminoverse’s UPO enzyme panel and identified thirteen active UPOs that generated over twenty distinct products, including coniferyl aldehyde, vanillin, and β-hydroxypropiovanillone, all of which are relevant to pharmaceuticals, flavours, and fragrances.
UPOs distinguish themselves through their ability to act on a wide substrate spectrum while performing multiple reaction types, such as alcohol oxidation, O-demethylation, and bond cleavage. This promiscuity makes them powerful discovery tools, enabling the development of novel chemistries and access to valuable molecules from renewable resources.
An enzyme collection like the Aminoverse UPO panel offers the advantage of accelerating identification of productive enzyme–substrate matches. This paves the way to scalable and sustainable synthesis of diverse aromatic building blocks.
Unlocking lignin valorisation: Oxyfunctionalization of lignin dimer model compounds by unspecific peroxygenases
“Lignin is an abundantly available biopolymer composed of three structural units, linked by a complex network of bonds, including a high proportion of β-O-4 ether linkages. As a renewable carbon source, it can be depolymerised into a variety of small aromatic compounds such as monophenols. Enzymatic bioprocessing offers a promising alternative to traditional chemical lignin degradation strategies, potentially producing value-added compounds, such as monoaromatics. Unspecific peroxygenases (UPOs) are promising enzymes for lignin bioprocessing due to their ability to catalyse aromatic oxidation and demethylation reactions, which are critical for lignin valorisation. In this study, thirteen different UPOs were evaluated for their oxidation potential with two lignin dimer model compounds, guaiacylglycerol-β-guaiacyl ether and veratrylglycerol-β-guaiacyl ether. Both compounds were successfully processed, yielding a wide range of products, e.g., via Cα-oxidation, demethylation, and bond cleavage reactions. Notably, the cleavages frequently occurred at the Cβ-O ether bond, a major linkage between the lignin monomers, being beneficial for lignin degradation and subsequent valorisation. Some of the identified products, such as vanillin, are of interest either as valuable end-products or as precursors for further conversion into specialty chemicals.”
