Scalable biocatalysis meets cosmetic innovation
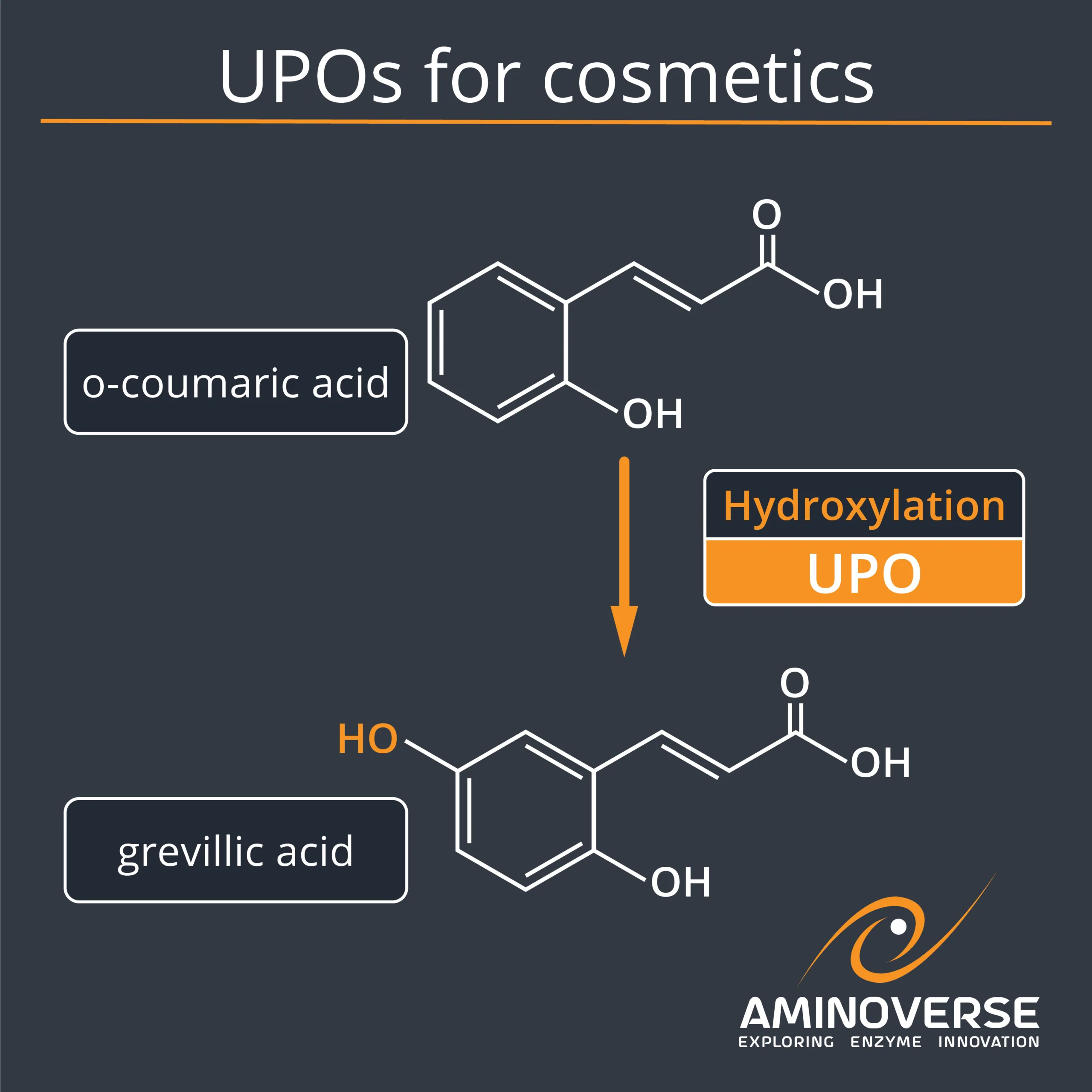
In a study by Clemens Cziegler, Benjamin Baumert, Christoffel P. S. Badenhorst, Karsten Siems, and Uwe T. Bornscheuer, the Aminoverse UPO kit proved instrumental in developing an efficient biosynthetic route to grevillic acid, a promising ingredient for cosmetics.
Screening with the Aminoverse 44 Kit enabled the team to assess solvent and temperature tolerance across multiple unspecific peroxygenases (UPOs). UPO2 emerged with superior temperature stability, guiding the team to refine conditions for optimal synthesis.
Grevillic acid is valued in cosmetics for its antioxidant properties and potential to support skin health. By securing a scalable biocatalytic process, the study demonstrates how Aminoverse UPOs help bridge sustainable production with industrial demand, making novel actives, such as grevillic acid, more accessible to formulators.
The findings highlight a promising approach to sustainable and selective biocatalysis for natural product synthesis.
Selective Enzymatic C−H-Oxyfunctionalization for the Efficient Synthesis of Grevillic Acid
“In this study, a selective C−H-oxyfunctionalization using an unspecific peroxygenase (UPO) enabled an efficient biosynthetic route for the synthesis of grevillic acid (GA), a natural antioxidant. The route commenced with the release of o-coumaric acid (oCA) from trans-2-coumaric acid glucoside (trans-CAG) using commercially available β-glucosidase from almonds. In addition, a cis-to-trans photoisomerization of cis-CAG was implemented to increase the synthetic access of oCA. The key step, the para-hydroxylation relative to the existing hydroxyl group of oCA, was accomplished by an UPO with full conversion and 93% isolated yield. Despite its name, the UPO turned out to exhibit excellent regioselectivity in this C−H functionalization, requiring only H2O2 as a cosubstrate.”
